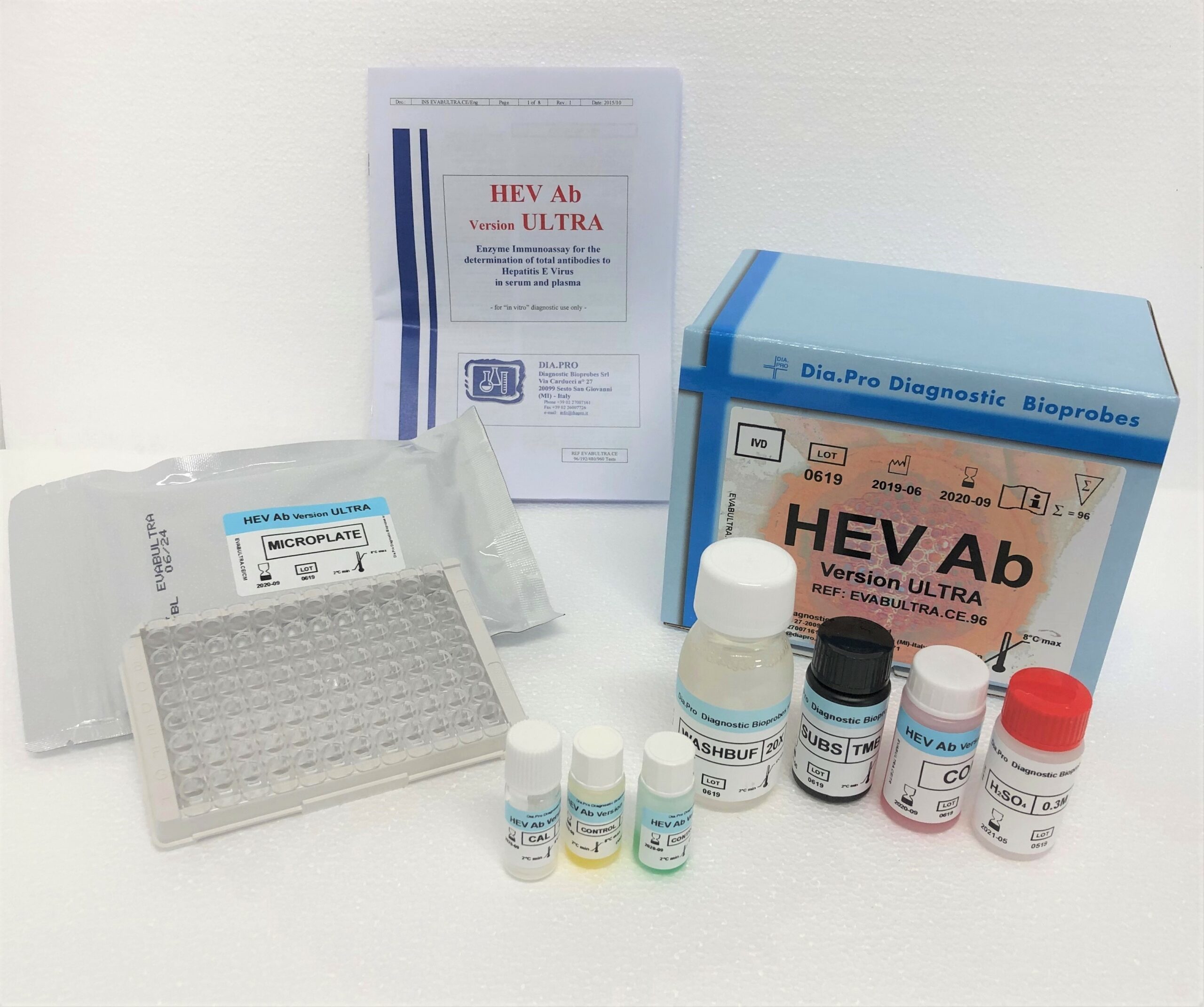
HEV Ab (Version ULTRA) – ELISA
Third generation Enzyme ImmunoAssay (ELISA) for the qualitative determination of total antibodies to Hepatitis E Virus (HEV) in serum and plasma. The kit is intended for the follow-up of HEV-infected patients and the screening of blood units.
In addition, due to the assay configuration of the product, the kit may be used also in testing total antibodies to HEV in serum and plasma derived from other not-human recipients for zoonotic studies.
For “in vitro” diagnostic use only.
Microplates are coated with highly specific synthetic antigen encoding for conservative and immunodominant determinants of HEV.
The solid phase is first treated with the sample where anti HEV total antibodies (mostly IgG, IgM and IgA) are captured, if present, by the antigens.
After washing out all the other components of the sample, in the second incubation bound anti HEV total antibodies are detected by the addition of the same HEV highly specific synthetic antigen labeled with peroxidase (HRP).
The enzyme captured on the solid phase, acting on the substrate/chromogen mixture, generates an optical signal that is proportional to the amount of anti HEV antibodies present in the sample. After blocking the enzymatic reaction, its optical density is measured by an ELISA reader.
The version ULTRA is particularly suitable for automated screenings.